Islam MD. Rafiqul
(6H-1,3,5-オキサカルコゲナジンからの窒素およびカルコゲン原子を持つ5員環ヘテロ環状化合物への新規な変換))
Recently, the enormous increase in synthesis of heterocyclic compounds containing chalcogen and nitrogen atoms has been leading to develop new methods. In this way, generation and synthetic application of 1,3-chalcogenaza-1,3-butadienes and their oxidized variants would be versatile building blocks because of their heterocumulene-like structure and potential reactivities. Within this earnest demand, only limited findings have been realized because of the preparative difficulties of their precursors and lack of suitable trapping methods for these highly reactive species. In the course of studies of highly-reactive chalcogenocarbonyl functionalities bearing π-conjugation systems, several novel methods were developed for the conversion of 6H-1,3,5-oxachacogenazines into five-membered heterocyclic compounds.
The following methods for the synthesis of five-membered heterocyclic compounds have been developed:
Preparation of 6H-1,3,5-Oxachalcogenazines and S-Oxides
6H-1,3,5-oxachalcogenazines 1 (X=S) and 2 (X=Se) were prepared as single stereoisomers by treating a thio- or selenoamide with an aldehyde and BF3.OEt2 according to the reported procedure. Oxidation of 1 by mCPBA gave the corresponding 6H-1,3,5-oxathiazine S-oxides 3 as single epimers in high yields.
Synthesis of 5H-1,2,4-Oxathiazoles 4 through Thermal Cycloreversion of 6H-1,3,5-Oxathiazine S-Oxides 3
Heating of a CHCl3 solution of 3 afforded hitherto unknown 5H-1,2,4-oxathiazoles 4 quantitatively. When a CDCl3 solution of 3a (R1=Ph, R2=t-Bu) was kept standing at 25 0C in an NMR tube, a mixture of 4 and pivalaldehyde was formed in a 1:1 molar ratio, suggesting that thermal cycloreversion of 3 would generate A, and which might subsequently cause facile ring closure to afford 4 (Scheme 1).
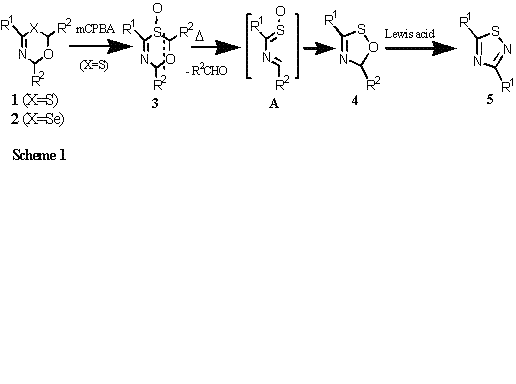
Compounds 4 were efficiently converted into 1,2,4-thidiazoles 5 through plausible pathway involving acid-induced hydrolytic fragmentation of 4 and the subsequent self-condensation of intermediacy, thioamide S-oxide. Novel Conversion of 6H-1,3,5-Oxathiazine S-oxides 3 into 3H-1,2,4-Dithiazoles 6 by the treatment with LR When 3 was heated with Lawesson's reagent (LR) at toluene refluxing temperature, 3H-1,2,4-dithiazoles 6 were afforded in moderate to high yields. An NMR monitoring experiment of 3a with LR in CDCl3 at 25 0C was observed quantitative formation of pivalaldehyde along with a trace amount of 6a. This result suggests that the reaction of 3a with LR might initially form intermediacy B, and the subsequent thermal cycloreversion of B would form C and which finally give 6 by eliminating PSOAnis moiety (Scheme 2). On the other hand, when 3 was treated with LR in the presence of EtOH, deoxygenation products 1 were formed.
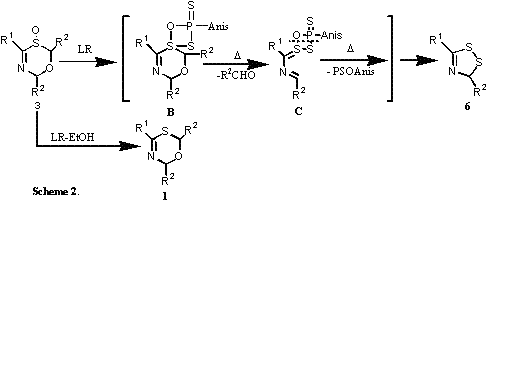
Synthesis of 3H-1,2,4-Dithiazoles, 3H-1,2,4-Thiaselenazoles and 3H-1,2,4-Diselenazoles
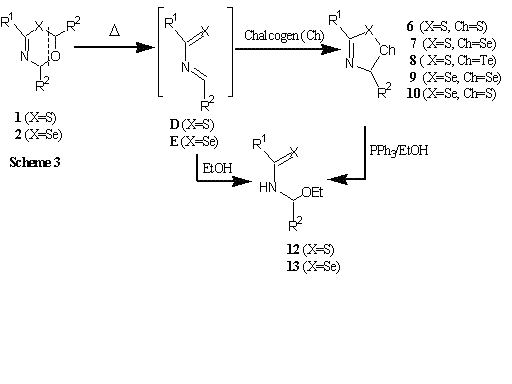
Five-membered heterocyclic compounds, 3H-1,2,4-dithiazoles 6 and 3H-1,2,4-thiaselenazoles
7 possessing S-S and S-Se bonds, were synthesized by the reaction of 1 with elemental sulfur or selenium,
respectively. On the other hand, 3H-1,2,4-diselenazoles 9 were prepared by the reaction of 2
with elemental selenium.
It can propose that the formation pathway of compounds 6, 7, 9 involving in situ generation of
heterodienes D or E through thermal cycloreversion of 1 or 2 followed by [4+1]-type cycloaddition of
heterodiene with sulfur or selenium species. Although compound 3H-1,2,4-thiatellurazoles 8 could
not isolate by the reaction of 1 with elemental tellurium, it was expected that compound 8 was
transiently formed which subsequently converted into 3H-1,2,4-dithiazole 6 via
Tellurium-Sulfur exchange. In the reaction of 2 with elemental sulfur, the formation of 5H
-1,2,4-thiaselenazole 10 was suggested by NMR data. However, compound 10 could not isolate due to
inseparable mixture with compounds 6 and 9.
Reactivity and synthetic utilities of compounds 6, 7, and 9 were also explored.
Oxidation of compounds 6 by mCPBA afforded the corresponding 3H-1,2,4-dithiazole S
-oxides 11 in which oxygen atom was selectively introduced onto S-2 sulfur.
Moreover, the relative stereochemistry of S-O moiety with t-Butyl group in compounds 11 was trans-orientation.
When 6, 7, and 9 were treated with Ph3P at room temperature, the S-2 sulfur and Se-2
selenium were extruded to generate 1,3-thiaza-1,3-butadienes D and 1,3-selenaza-1,3-butadienes E,
which were efficiently trapped by EtOH to give the corresponding 1,4-adducts 12 and 13, respectively.
In conclusion, a novel method was developed for the synthesis of 5H-1,2,4-oxathiazoles 4 through thermal cycloreversion of 6H-1,3,5-oxathiazine S-oxides 3. A novel conversion of 3 into 6 by the treatment of LR was realized. A wide range of 5-membered 1,2,4-dichalcogenazoles 6-10 were also synthesized by the reaction of in situ generated 1,3-chalcogenaza-1,3-butadienes with elemental chalcogen.